- General Dermatology
- Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
Coppertone Recalls 12 Lots of Aerosol Sunscreen
Coppertone has issued a voluntary recall for 12 lots of its aerosol sunscreen, citing detected levels of the carcinogen benzene.
Affected Coppertone products

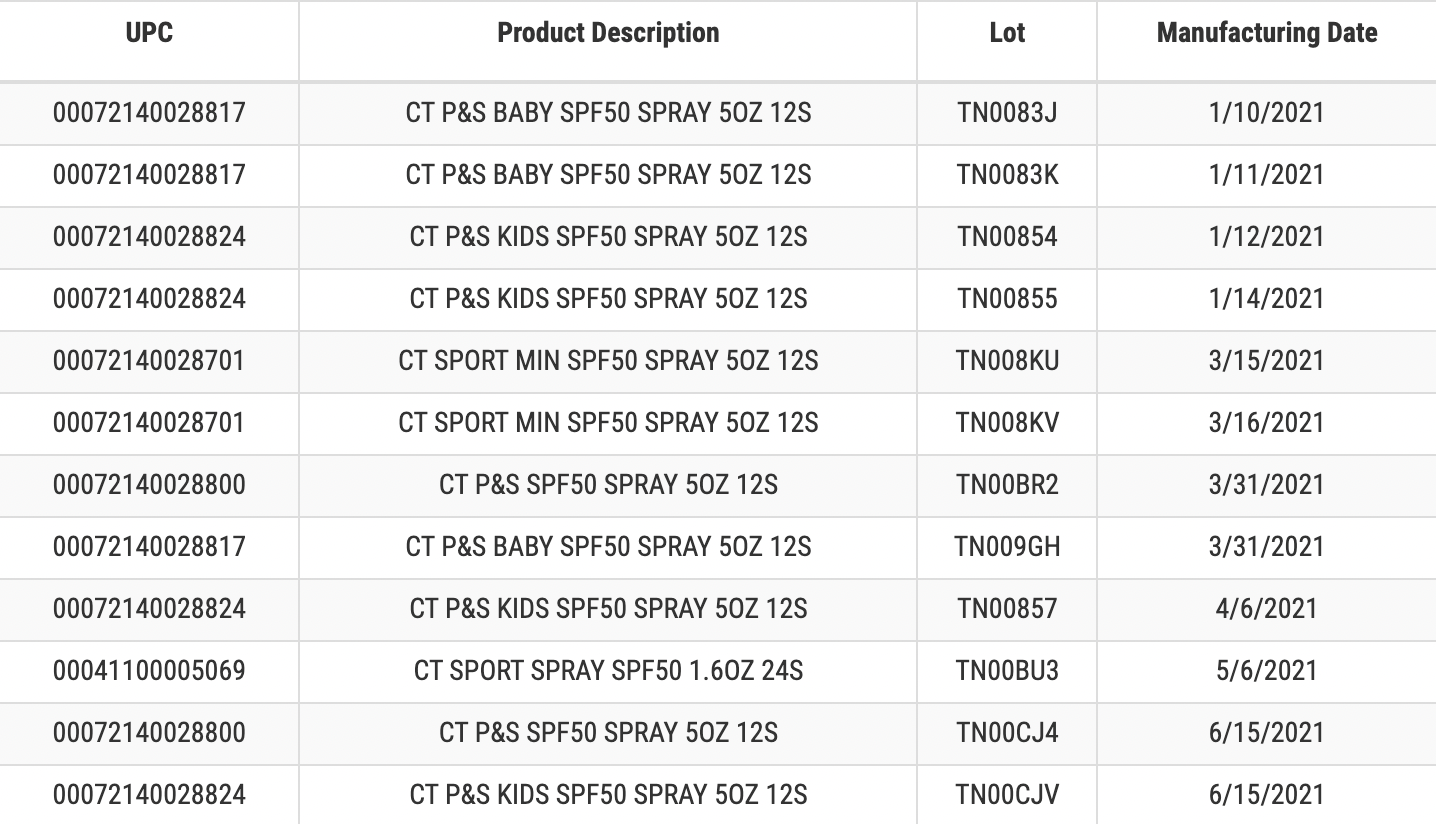
Coppertone has issued a voluntary recall for 12 lots of its aerosol sunscreen products manufactured between January 10, 2021 and June 15, 2021, citing the presence of benzene, a human carcinogen, in these lots of sunscreen products.
The recalled products include:
Table courtesy of the FDA.
The FDA advises consumers to stop using these products and dispose of these appropriately.
Benzene is classified as a human carcinogen, according to the FDA. Exposure to this carcinogen can happen through inhalation, orally, and through the skin. Also, depending on the extent and level of exposure, benzene exposure could lead to an increased risk of blood cancers such as leukemia, blood cancer of the bone marrow and other blood disorders.
The FDA states that daily exposure to benzene at the levels detected in these affected aerosol sunscreen products are not be expected to cause any adverse health effects, citing generally accepted exposure modeling by numerous regulatory agencies. They also note, to date, Coppertone has not received any reports of adverse events related to this voluntary recall.
Based on generally accepted exposure modeling by numerous regulatory agencies, daily exposure to benzene levels detected in the Coppertone aerosol sunscreen sprays that are part of this voluntary recall would not be expected to cause adverse health consequences; however, consumers should stop using these specific Coppertone aerosol sunscreen spray products that are part of this voluntary recall and dispose of them appropriately.
- Compliance with wearing sunscreen has long been issue. What guidance can you offer on dermatologists reassure patients that sunscreen plays a key role not only in skin cancer prevention but also to help prevent flares of some inflammatory skin conditions?
While we cannot provide medical advice to dermatologists or their patients, we certainly agree with the FDA and other medical authorities on the importance of using of sunscreen, along with other protective measures.
- How soon will packaging change to reflect the Sept. 24 FDA announcement regarding ingredient listing placement?
In the most recent FDA announcement, there are overall labeling changes proposed impacting the front and back panels of sunscreen labels. These proposed changes will have a comment period of 45 days, then go into effect a year after the final order is issued.
Regarding your question on Coppertone products contained in our FDA Citizen Petition, only one Coppertone product Valisure analyzed had traces of benzene below 0.1ppm and the other ten products had no detectable benzene. However, we didn't have the opportunity to test any of the sunscreen products that are currently part of the Coppertone recall. This underscores how complex the manufacturer supply chains are, even within a single company. I also found the recent Coppertone sunscreen recalls interesting from the perspective that they mostly involved mineral based products where zinc oxide is the active ingredient. There doesn't appear to be a mechanism where the zinc could be the source of benzene, so it serves as further evidence that raw materials or inactive ingredients are likely the source of the contamination.
Regarding FDA actions, we certainly hope these and other recent recalls for benzene contamination prompts the FDA to take more action on this overall issue with consumer healthcare products, both OTC drugs and cosmetics. We are still waiting for FDA to fill the current regulatory gap on benzene where a limit of 2 ppm is only defined for emergency situations and drug products that constitute a "significant therapeutic advance" and require benzene for their manufacture; none of which appears to apply to sunscreen. Furthermore, as Dr. Bunick has emphasized, even a very low concentration of benzene in a product like sunscreen quickly adds up to a very high total exposure, since the proper application of sunscreen can use high volumes of product. Therefore, a daily exposure limit for benzene in standard products needs to be established.
More information on the product recall, including images, lot information, and refund requests can be found at www.sunscreenrecall2021.com.
